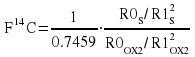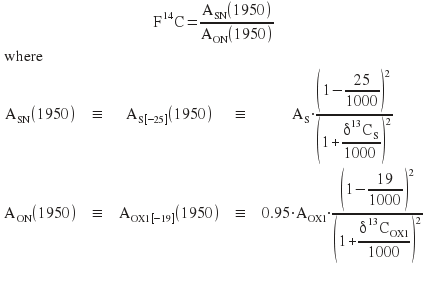
We start with the Stuiver notation. A is the 14C/12C activity and subscripts S, OX refer to Sample and Oxalic respectively. Subscript N refers to a fractionation corrected quantity, but it will be dropped after the 2nd equation in favour of using [] which enclose the value to which the fractionation correction is made. Once the sample has been age corrected to 1950 the (1950) can also be dropped because the sample and the standard are measured at the same time.
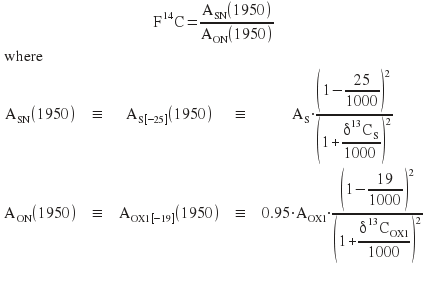
First of all, we rewrite the equations using a normalization to OX2 instead of OX1. Stuiver (1983) relates the activity of OX1 and OX2 (his NOX) using
![]()
Therefore,
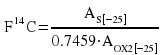
Because OX2 has δ13C of -17.8 relative to PDB, we use another Stuiver (1983) result:
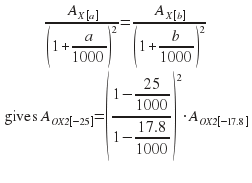
Then
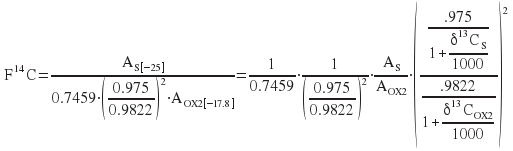
Introducing the notation
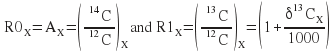
where zero and one refer to the rare and abundant isotope in the notation favoured by NEC, we finally have